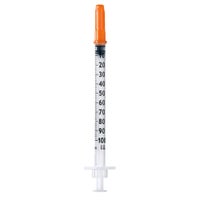
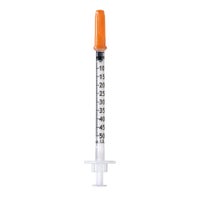
Omnican® 40 (Type LDS)
3-piece insulin syringes for U-40 insulin with integrated needle (type Low Dead Space)
Omnican® 40 is a hypodermic 3-piece insulin syringe for U-40 insulin with integrated needle (type LDS). The single-use device is intended to be used for the following purposes:
Aspiration and subcutaneous injection of an insulin, a hormone or medicinal solution following the dedicated scale graduation.
Admixture of insulin or other hormone or medicinal solution following the dedicated scale graduation.
The devices can be used for all patients for which infusion therapy is prescribed. No gender or age related limitations. Omnican® can be used for adults and pediatrics.
The syringe barrel is made of polypropylene and the plunger is made of polystyrene. The plunger stopper with double sealing ring is made of polyisoprene and the protective cap is made of polyethylene.
Advantages
- Fine 1 I.U. graduation
- Highly transparent barrel with high-contrast black graduation for easy reading and exact dosing
- Integrated thin-wall needle (according to EN ISO 9626)
- Lowest dead space syringe within the B. Braun syringe portfolio: reduced insulin loss due to integrated fine needle
- Barely perceptible puncture of skin due to precise three-facet bevel and silicone finish
- Highly transparent barrel to make sure that air bubbles are easily identified
- Safe plunger backstop helps to prevent unintentional plunger withdrawal and the loss of any liquids
- Red protection cap
- Not manufactured with Polyvinylchloride (PVC), Bisphenol A (BPA), Diethylhexylphthalate (DEHP) and Latex
- Manufactured according to ISO 8537
In addition the integrated needle features the following user benefits:
- Allows for minimal residual volume
- Higher number of doses that can be extracted out of a multi-dose vial compared to a standard syringe
- Reduction of drug loss
- Avoidance of additional costs
- No change in current clinical practive when using Low Dead Space syringes
Handling steps Omnican® insulin syringes
Animation